EUDAMED is the European Medical Device Databank. It’s a safe, web-based portal that serves as a hub for information exchange between national authorities and the European Commission. The portal is not accessible to the public. EUDAMED came into force in May 2011 intending to improve market surveillance and transparency in the setup of medical devices on markets by making it easier for anyone who wants access. The EUDAMED public website’s official URL is “ec.europa.eu/tools/eudamed”.
It is interoperable and serves as a registration system, a collaborative system, a notification system, and a dissemination system (open to the public).
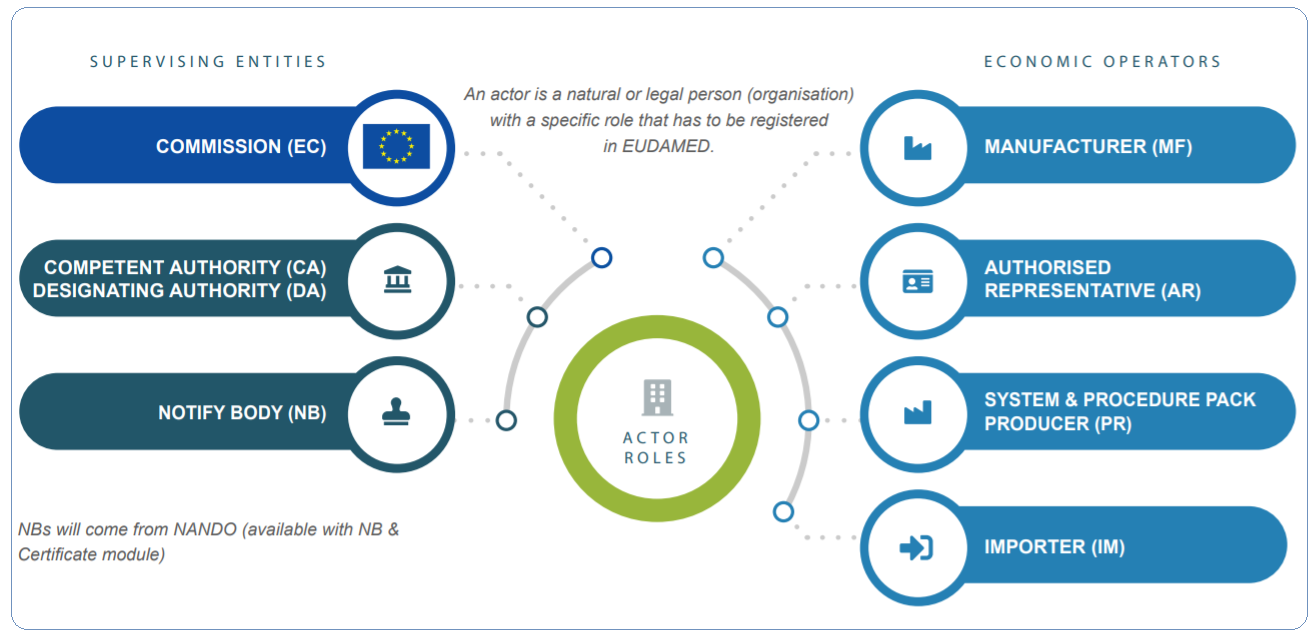
All economic operators in the European territory and non-EU manufacturers, authorized representatives, system/procedure pack producers, and importers must register as an actor in EUDAMED and provide the required data. The actor registration module of the European Commission’s Eudamed database, which is a critical component of the EU’s Medical Devices Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR),
Economic operators established in the EU or with authorized representation in the EU can now register. Economic operators headquartered in the United Kingdom, Switzerland, and Turkey will be unable to register unless agreements are negotiated between those countries and the EU.
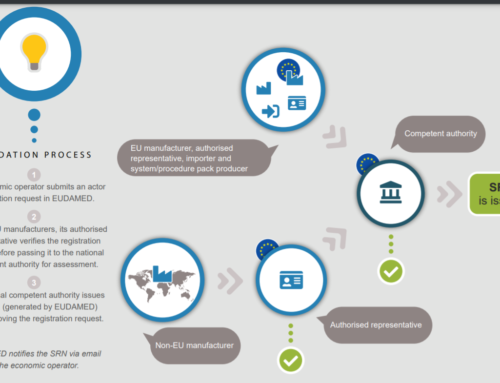
The actor module, which is required to access and use EUDAMED, enables economic operators to receive a single registration number (SRN), which serves as an EU-wide unique identity. The EUDAMED actor module also includes a searchable database of registered economic operators and competent authorities, allowing users to get information about the actors, such as contact information and identity, as well as information on the firms’ accountable individuals for regulatory compliance.
EUDAMED is built on six interconnected modules and a public website:
- Actors Registration- The process of registering an organization or individual in the EUDAMED database is known as EUDAMED actor registration. This is necessary in order to access certain portal features, such as submitting clinical trial applications or placing devices on the market.
- Device registration / UDI – All medical devices that are placed on the market must have a UDI. The EUDAMED UDI/device registration module is a centralized EUDAMED database that contains data on every medical device with a UDI that is placed on the European market.
- Certificates and Notified Bodies – The EUDAMED regulation requires medical devices to meet safety and performance standards. Certificates issued by notified bodies are accepted across Europe.
- Clinical Investigations and performance studies – Clinical studies must be carried out in accordance with the EU Clinical Investigation Requirements. This includes performance studies that are carried out to demonstrate a medical device’s safety and performance. That information is also included in the Clinical Evaluation information.
- Post-market surveillance and vigilance – post-market surveillance is required for medical devices. This includes keeping an eye out for negative events and complaints, as well as product recalls.
- Market Surveillance- Competent authorities conduct market surveillance activities to ensure that EU medical device requirements are met.
The use of EUDAMED is neither mandatory nor required at this time. Some modules are already available and can be freely used. Their use, however, cannot be compelled. Particularly:
- Since December 2020, the Actor Registration Module has been available for voluntary use.
- Since October 2021, the UDI/device registration module has been available for voluntary use.
- Except for the mechanisms for scrutiny and the clinical evaluation consultation procedure (CECP), the module on Notified Bodies and Certificates has been available for voluntary use since October 2021.
- The remaining modules, Vigilance, Clinical Investigation & Performance Studies, and Market Surveillance are in the works and will be made available once the entire EUDAMED system (including all six modules) is declared fully functional.
EUDAMED stores a wide range of data, including:
- Clinical Investigations Information
- A copy of the ISO certificate/QMS proof
- Justification of classification in accordance with Annex IX
- An ISO certificate or a vigilance and traceability system
- Declaration of conformity
- Manufacturers, devices, and Authorized Representatives must all be registered.
- Any additional documentation
EUDAMED also stores data from clinical investigations and performance studies. This includes information on the study’s design, methodology, and results. When applying for CE Marking for their devices, manufacturers must include this information.
Note: Image source: https://ec.europa.eu/