MEDICAL DEVICE BIOLOGICAL EVALUATION
Biological Evaluation of Medical Devices or biocompatibility testing evaluates the compatibility of medical devices with a biological system and is an important element of biological risk assessment. It investigates how the device interacts with the many types of live tissues and cells exposed to it when it comes into touch with patients.
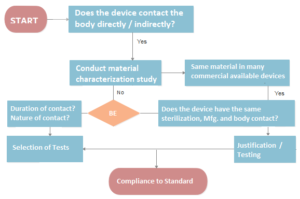
The toxicological assessments and the selection of device materials to be tested should take into consideration thorough characterization of all manufacturing materials.
The device’s manufacturing materials, end product, and potentially leachable chemicals or degradation products should all be examined for their impact on the device’s overall toxicological assessment.
The bioavailability of the substance should be considered in the tests used in the toxicological evaluation (i.e., nature, degree, frequency, duration and conditions of exposure of the device to the body).
Any in vitro or in vivo research or testing should follow established Good Laboratory Practice guidelines (GLP).
Unless testing is done pursuant to a recognised standard that does not require data submission, the whole experimental data should be given to the reviewing body.
Any change in the device’s chemical composition, manufacturing method, physical configuration, or intended usage should be assessed for potential toxicological consequences and the need for further toxicity testing.
For an overall safety assessment, the toxicological examination done in line with this advice should be evaluated in connection with additional information from non-clinical testing, clinical trials, and post-market experiences.
BIOCOMPATABLITY TEST
Biological Evaluation of Medical Devices is critical to determine whether your product contains toxins or has the potential to cause harm. Our team of medical device regulatory experts along with lab toxicologists collaborate with you to plan the biocompatibility test identification and also helps with any justification if required.
Biological Evaluation of Medical Devices or safety has become more of a priority for Notified Bodies, as a result of the MDR and the 2020 amendment of ISO 10993-1. Learn how to utilise ISO 10993-1:2020 as a method to assess a medical device’s biological safety and how to arrange the biological assessment in a three-tiered manner.

Bacterial reverse mutation (Ames) test – Genotoxicity
Chromosomal aberration test – Genotoxicity
Hemocompatibility: Partial thromboplastin time – PTT
Hemocompatibility: (Platelets and Leucocytes)
Hemocompatibility test: Platelet Activation Test
Hemolysis test (Direct & Indirect contact method)
Cytotoxicity Test – MEM Elution method
Intramuscular (4 week’s) implantation Test
Skin sensitization test (GPMT method)
Intracutaneous reactivity test
Acute systemic toxicity test
Material mediated pyrogen test
Subacute (28 days) systemic toxicity test
Part 17 is very important in the Biological Evaluation of Medical Devices as per the new MDR.
More details will be updated soon.
Part 18 is very important in the Biological Evaluation of Medical Devices as per the new MDR.
More details will be updated soon.
BIOLOGICAL EVALUATION PROCEDURE
BIOLOGICAL EVALUATION PLAN
The whole collection of papers for the biological evaluation of medical devices may be obtained online. These documents are in easy-to-edit word documents.