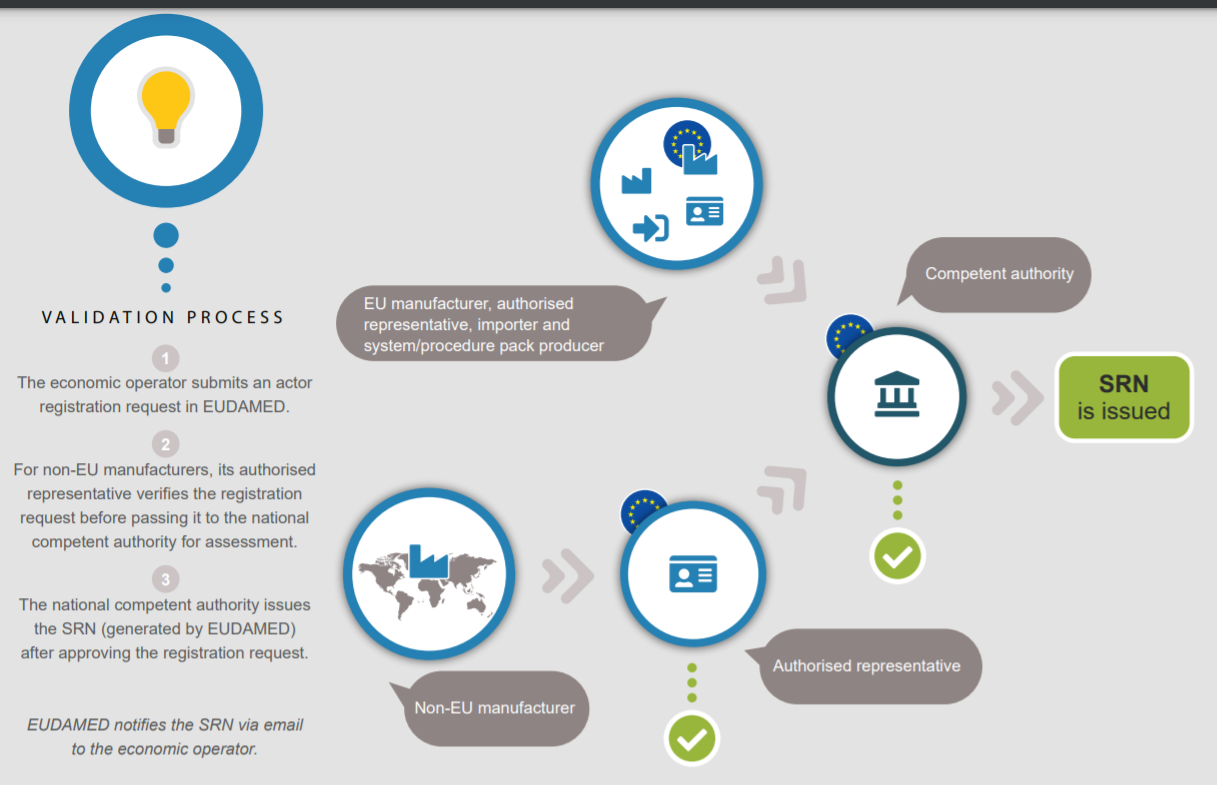
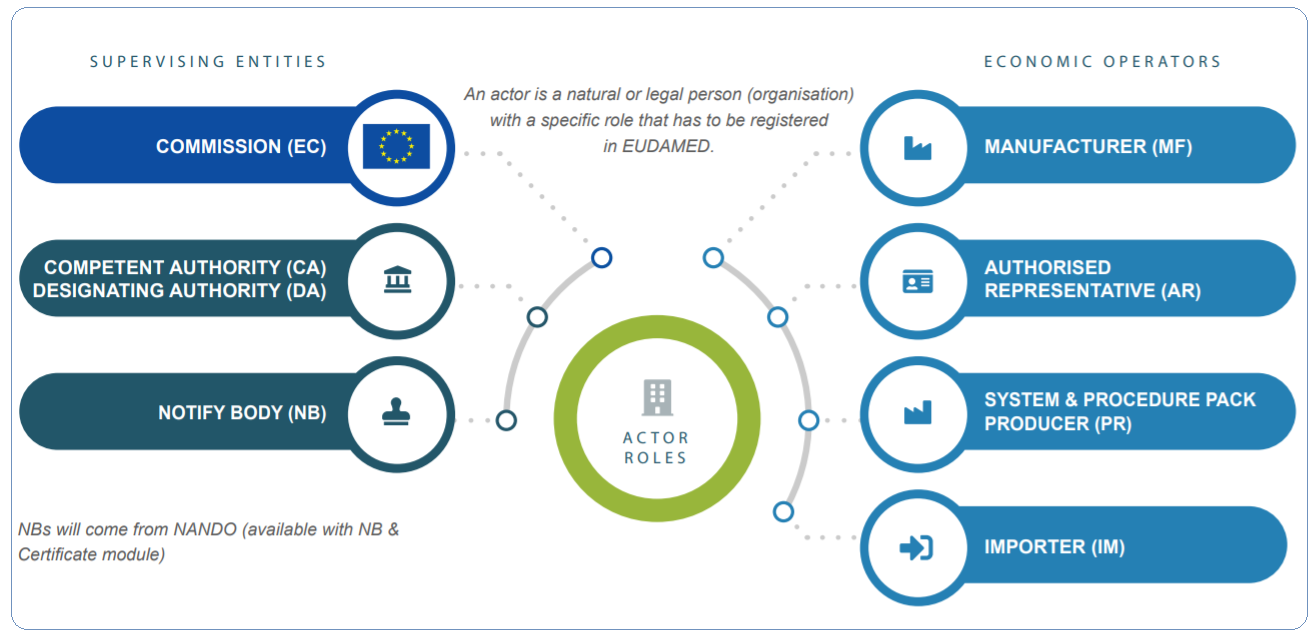
MEDICAL DEVICE CLINICAL EVALUATION : AN OVERVIEW
M edical Device Clinical Evaluation is a procedure to collect, assess, analyze a clinical data based on a clinical device to analyze whether there is sufficient clinical evidence to confirm compliance with essential safety and performance requirements when using according to manufacture instruction of use.it is applicable to all classes of medical device based [...]