What exactly is a Single Registration Number (SRN)?
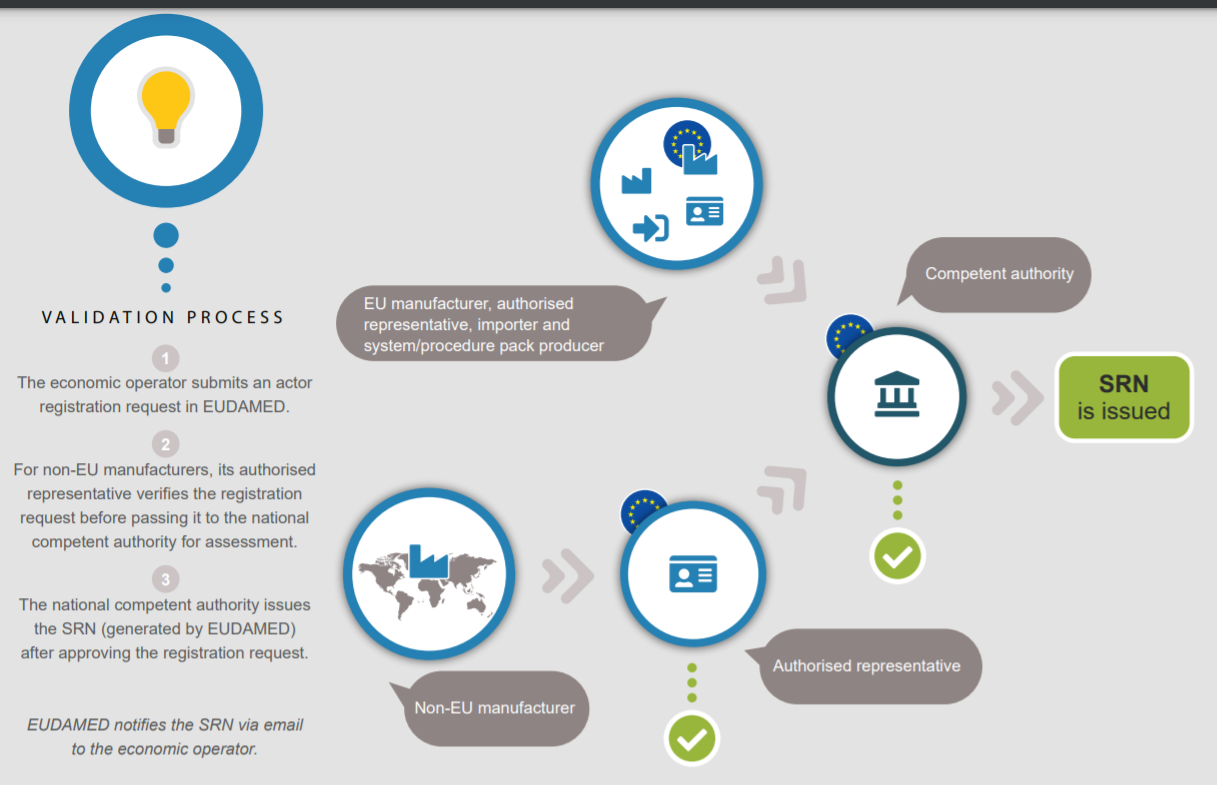
The Single Registration Number (SRN) is a unique code that is used to identify an economic operator in the EU without ambiguity. The SRN number is generated by EUDAMED and issued by the competent authority who validated the Actor registration request in EUDAMED. Manufacturers must apply for the SRN number any time now! No need to wait for EUDAMED to be fully functional. The latest estimate is that the deadline will be November 26, 2022.
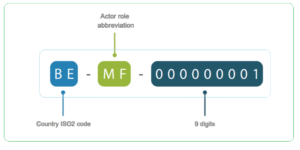
HOW TO READ SRN?
XX – Country Code
XX – two-letter abbreviation of the actor’s role
XXXXXXXXX – 9-digit number code auto generated by system for the applicant
Prior to the product being marketed, the Competent Authority must grant the SRN. Failure to meet these requirements will almost certainly result in market authorization being rejected or denied.
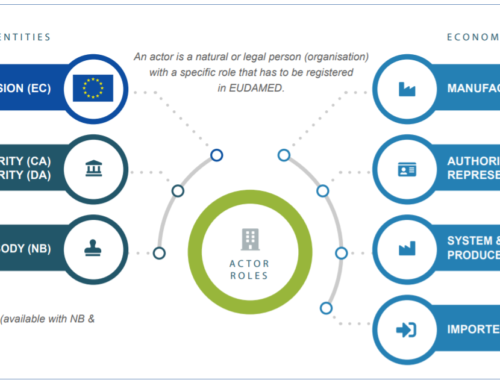
WHO NEEDS AN SRN?
All economic operators or actors subject to Regulations 2017/745 (MDR) and/or 2017/746 (MDR) must register in the Actor Module of EUDAMED
The following actors and roles require registration in SRN;
- The manufacturer (or legal manufacturer)
- Authorized Representative
- Producer of the Authorized Representative (EC REP)
- System and Procedure Pack
- EU Importer
Distributors, on the other hand, are not required to register with EUDAMED.
We are regulatory experts with a proven track record of successfully completing projects in the medical device industry. For assistance, please contact us.
Related –