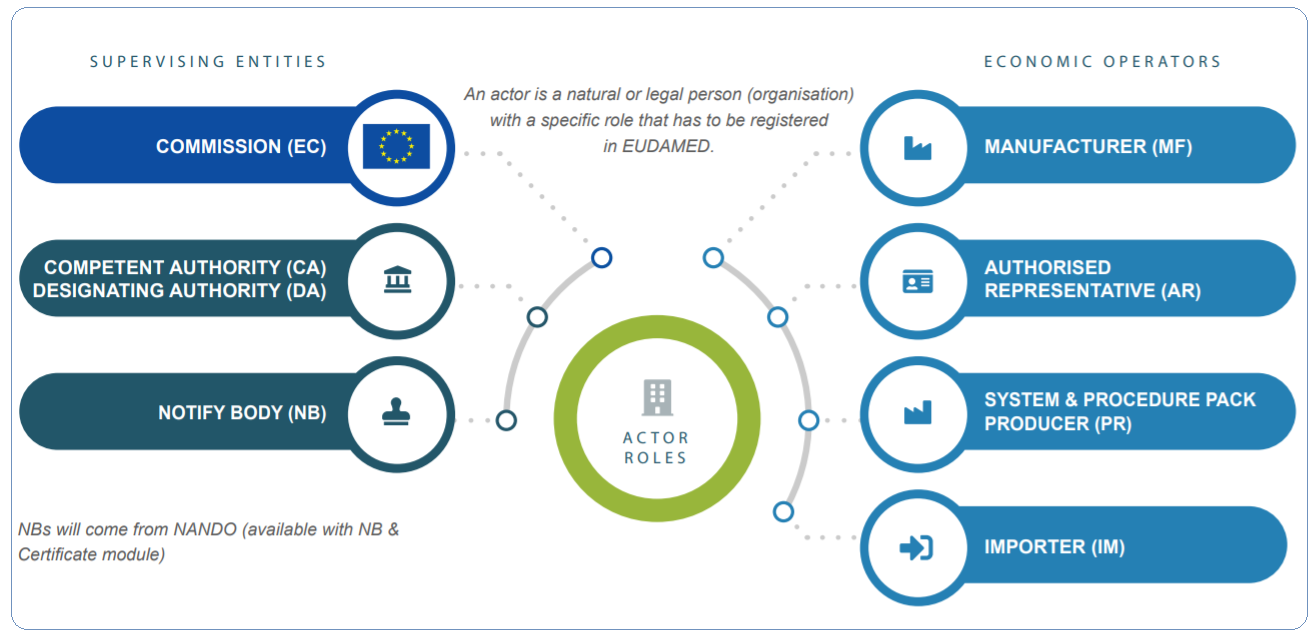
Eudamed Actor Registration
EUDAMED is the European Medical Device Databank. It's a safe, web-based portal that serves as a hub for information exchange between national authorities and the European Commission. The portal is not accessible to the public. EUDAMED came into force in May 2011 intending to improve market surveillance and transparency in the setup of medical [...]