From Testing to BER – Your Partner in ISO 10993 Compliance.
Biological Evaluation for Medical Devices
Biological evaluation is a critical component of medical device safety assessment, required under global regulations such as the EU MDR and FDA guidelines. It ensures that a device does not pose any unacceptable biological risk to patients or users when in contact with the human body. This process follows the framework of ISO 10993, the international standard for evaluating the biocompatibility of medical devices.
At RegHelps, we assist manufacturers in preparing comprehensive Biological Evaluation Reports (BERs) by identifying relevant biological endpoints, conducting or reviewing biocompatibility testing, and performing toxicological risk assessments. Whether your device is skin-contacting or implanted long-term, our team ensures that all evaluations are evidence-based and meet Notified Body or FDA expectations.
Our support covers:
Biocompatability Test
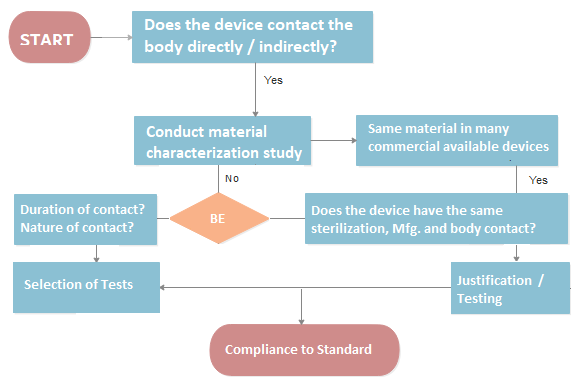
Biological Evaluation of Medical Devices is critical to determine whether your product contains toxins or has the potential to cause harm. Our team of medical device regulatory experts along with lab toxicologists collaborate with you to plan the biocompatibility test identification and also helps with any justification if required.
Biological Evaluation of Medical Devices or safety has become more of a priority for Notified Bodies, as a result of the MDR and the 2020 amendment of ISO 10993-1. Learn how to utilise ISO 10993-1:2020 as a method to assess a medical device’s biological safety and how to arrange the biological assessment in a three-tiered manner.
service related FAQ’s
Not always. If sufficient historical data or literature exists to demonstrate the safety of materials used in the device, some tests may be waived. A gap analysis helps determine whether existing data is adequate or if additional testing (e.g., cytotoxicity, irritation, sensitization) is required to meet regulatory expectations.
Its highly recommended to conduct testing of finished salable device before you apply for regulatory clearance.
A Biological Evaluation Report (BER) summarizes the device’s biological risk assessment, including device description, material characterization, exposure categorization, literature review, testing results (if applicable), and a toxicological risk assessment. It concludes whether the device is biologically safe and compliant with ISO 10993.

