
Stay compliant. Protect your CE Mark. Maintain patient safety.
Post-Market Clinical Follow-Up (PMCF) Services for MDR CE Certification
Under EU MDR 2017/745, Post-Market Clinical Follow-Up (PMCF) is an essential and ongoing process for all CE marked medical devices. It ensures that your device continues to meet safety, performance, and benefit–risk requirements throughout its entire life cycle.
At RegHelps, we take the complexity out of PMCF by offering end-to-end support from planning execution and reporting for onward submission by client to Notified Body helping you maintain MDR CE Certificate validity
Failure to maintain a robust PMCF program can result in non-conformities, suspended CE marking, and loss of EU market access. Following are the major reason for its importance.
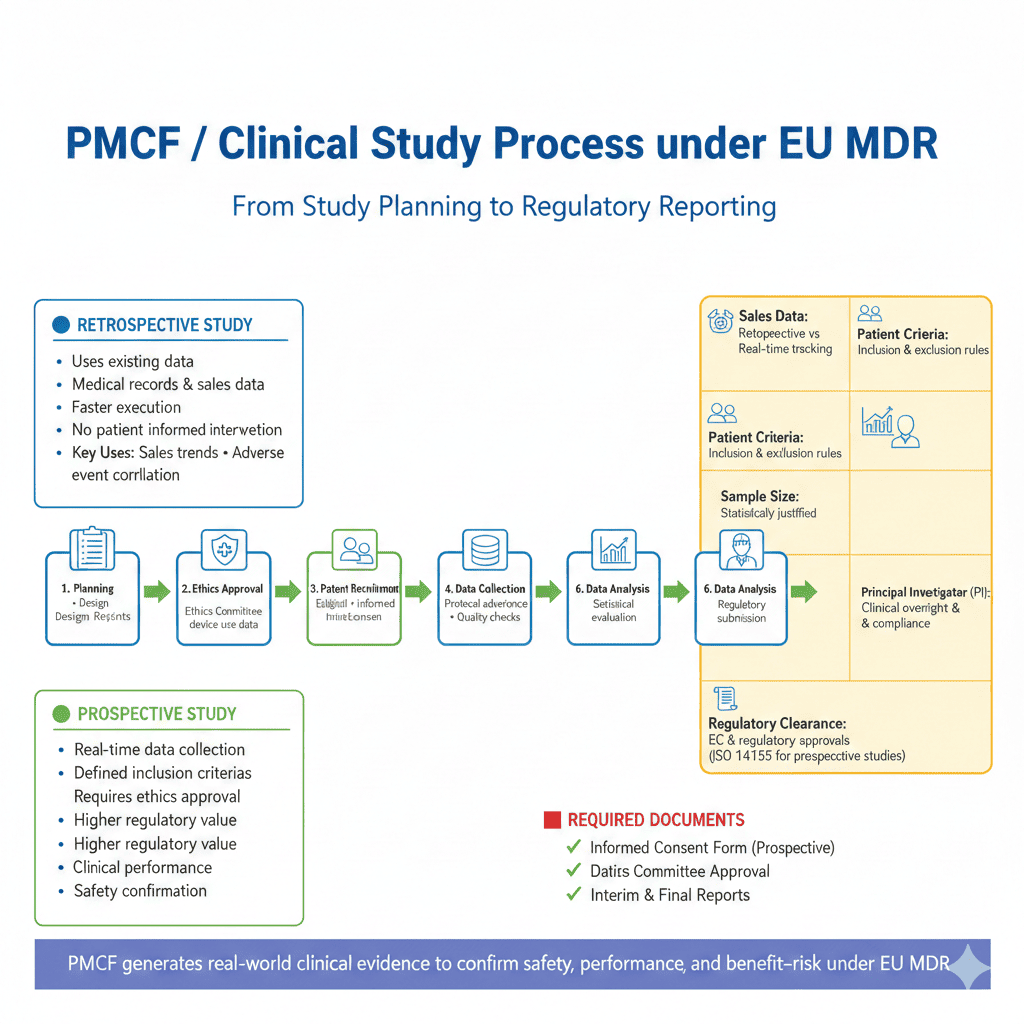
How RegHelps Supports Manufacturers
PMCF Plan Development
We prepare MDR-compliant PMCF Plans (Annex XIV, Part B) that clearly outline:
-
Objectives and clinical questions.
-
Data sources (surveys, registries, literature, clinical studies).
-
Timelines and responsibilities.
-
Integration with your overall Post-Market Surveillance (PMS) system.
PMCF Execution & Data Collection
We manage the complete execution of PMCF activities, including:
-
Clinical data collection from hospitals, clinics, and users.
-
Registry data monitoring for real-world performance trends.
-
Surveys and user feedback for device usability and safety insights.
-
Targeted clinical investigations where additional data is needed.
Literature & Database Monitoring
-
Continuous monitoring of scientific publications and clinical databases.
-
Adverse event analysis using sources like Eudamed and MAUDE.
-
Evidence updates for your CER and PMS documentation.
Data Analysis & Risk Updates
-
Statistical evaluation of collected data to identify trends or safety concerns.
-
Updates to your ISO 14971 Risk Management File.
-
Recommendations for corrective or preventive actions if needed.
PMCF Evaluation Report & PSUR Support
We prepare the PMCF Evaluation Report, summarizing:
-
Methods used and data sources.
-
Key findings on safety, performance, and benefit–risk.
-
Actions taken or planned for improvement.
For Class IIa, IIb, and III devices, this report feeds directly into your Periodic Safety Update Report (PSUR).
Why Choose RegHelps?
-
MDR Expertise – Experienced CRO with in-depth knowledge of MDR and Notified Body expectations.
-
Global Standards – Our PMCF processes align with ISO 14155 and ISO 14971.
-
Full Compliance Support – From planning to NB submission.
-
Time-Saving – We handle the complex documentation so you can focus on your business.
service related FAQ’s
Yes. Under EU MDR 2017/745, PMCF is a mandatory and ongoing activity for all CE-marked medical devices, regardless of risk class. The extent of PMCF activities may vary depending on the device’s risk profile, clinical history, and available data.
Absolutely. We can take over PMCF activities for devices already CE-marked and on the EU market, ensuring that your data collection, evaluation, and reporting fully meet MDR requirements — even if your existing documentation needs corrective action before the next Notified Body audit.

