
Performance Evaluation Done Right, CE Certification Secured.
IVDR Performance Evaluation: Your Path to Compliance
This page provides a comprehensive overview of the new IVDR Performance Evaluation requirements under the new 2017/746 In Vitro Diagnostic Regulation and how IVDR Consultants can assist IVD manufacturers in navigating this complex process. We offer expert guidance and support to ensure your IVDs meet the necessary standards for market access.
Understanding IVDR Performance Evaluation
The IVDR places significant emphasis on demonstrating the performance of In Vitro Diagnostic (IVD) devices. Performance Evaluation is a systematic and documented process that establishes and maintains the analytical and clinical performance, and where applicable, the scientific validity of an IVD device. This evaluation is crucial for obtaining and maintaining CE marking, allowing your device to be legally marketed within the European Union.
The Performance Evaluation process involves gathering and appraising data to demonstrate that the IVD device achieves its intended purpose and meets the specified performance requirements. This data can come from various sources, including:
The IVDR Performance Evaluation Process: A Step-by-Step Guide
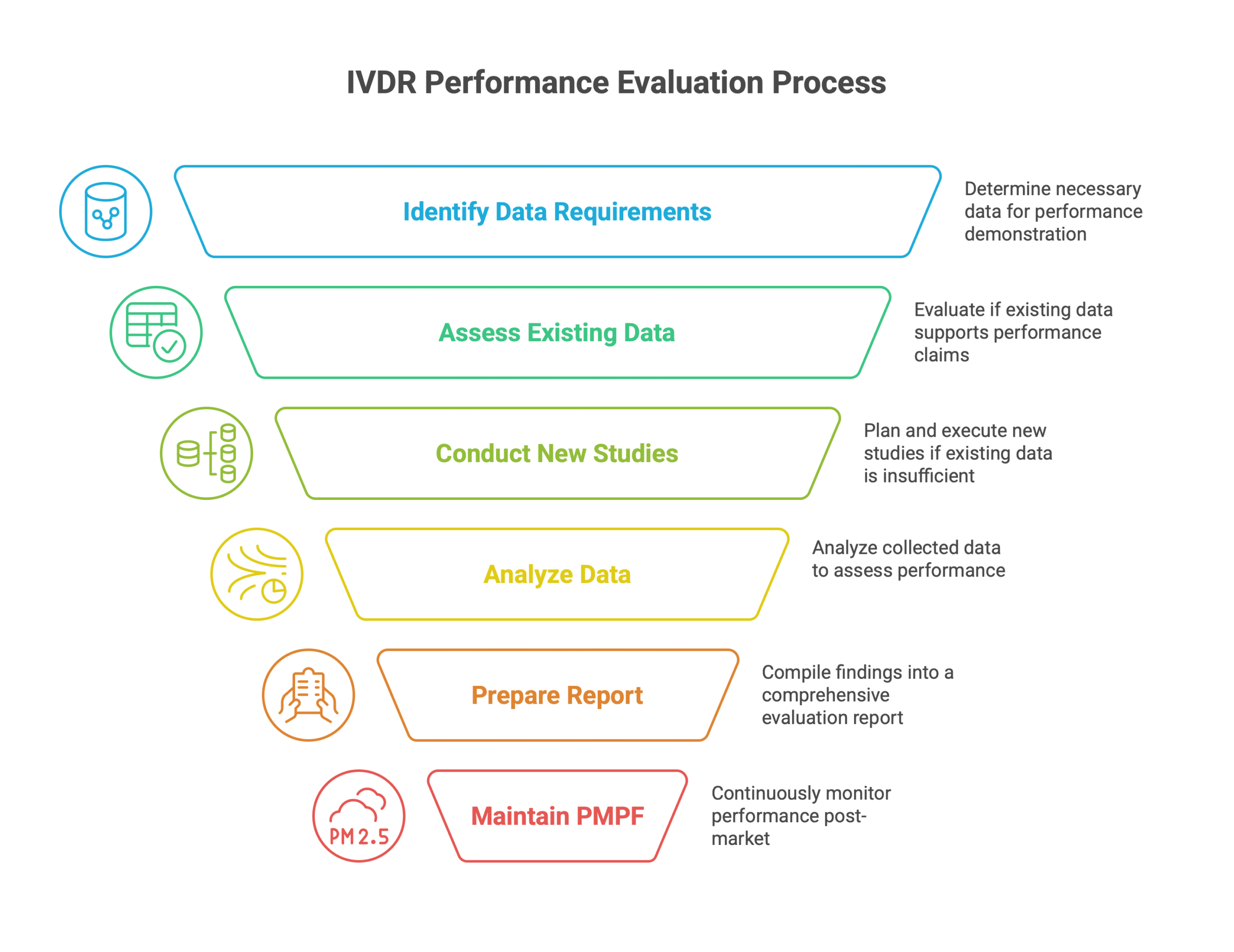
Explanation of the Steps:
-
Define Intended Purpose & Claims: Clearly define the intended purpose of the IVD device and the specific claims being made about its performance. This forms the basis for the entire evaluation.
-
Identify Data Requirements: Determine the specific data required to demonstrate the device’s performance based on its intended purpose and claims. This includes identifying the necessary analytical and clinical performance characteristics.
-
Existing Data Sufficient?: Assess whether existing data (e.g., literature, previous studies) is sufficient to support the performance claims.
-
Gather & Appraise Existing Data: If existing data is sufficient, gather and critically appraise its quality and relevance to the device’s intended purpose.
-
Plan & Conduct New Studies: If existing data is insufficient, plan and conduct new analytical and/or clinical performance studies to generate the necessary data.
-
Analyze Study Data: Analyze the data from both existing sources and new studies to determine whether the device meets the specified performance requirements.
-
Performance Acceptable?: Evaluate whether the analyzed data demonstrates that the device achieves its intended performance and meets the acceptance criteria.
-
Prepare Performance Evaluation Report: Compile all the data, methodology, results, and conclusions into a comprehensive Performance Evaluation Report (PER).
-
Iterate & Improve Device/Studies: If the performance is not acceptable, iterate on the device design, manufacturing process, or study protocols to improve performance.
-
Maintain PMPF: After the device is placed on the market, maintain a Post-Market Performance Follow-up (PMPF) system to continuously monitor its performance in the real-world setting.
-
Ongoing Performance Acceptable?: Continuously evaluate the data collected through PMPF to ensure that the device’s performance remains acceptable over time.
-
Continue Monitoring: If the ongoing performance is acceptable, continue monitoring the device’s performance through PMPF.
How RegHelps Team Can Help
Navigating the IVDR Performance Evaluation requirements can be challenging, especially for manufacturers with limited resources or expertise. IVDR Consultants offer a range of services to support you throughout the process, including:
-
Gap Analysis: Assessing your current documentation and processes to identify gaps in compliance with IVDR Performance Evaluation requirements.
-
Performance Evaluation Planning: Developing a comprehensive Performance Evaluation Plan that outlines the strategy for demonstrating the device’s performance.
-
Study Design & Management: Designing and managing analytical and clinical performance studies to generate the necessary data.
-
Data Analysis & Interpretation: Analyzing and interpreting data from existing sources and new studies to determine whether the device meets the specified performance requirements.
-
Performance Evaluation Report (PER) Writing: Preparing a comprehensive and compliant PER that summarizes the entire performance evaluation process.
-
Post-Market Performance Follow-up (PMPF) Support: Establishing and maintaining a PMPF system to continuously monitor the device’s performance in the real-world setting.
-
Scientific Validity Report Writing: Preparing a comprehensive and compliant Scientific Validity Report that demonstrates the scientific rationale for the analyte or marker being measured and its connection to the clinical condition or physiological state.
-
Regulatory Submission Support: Assisting with the preparation and submission of regulatory documents to Notified Bodies.
By partnering with RegHelps SRC, you can ensure that your IVD devices meet the IVDR Performance Evaluation requirements and gain access to the European market. We bring expertise, efficiency, and a deep understanding of the regulatory landscape to help you achieve your compliance goals. Contact us today to learn more about how we can support your IVDR journey.
MDCG 2022-2 Guidance on general principles of clinical evidence for In Vitro Diagnostic medical devices (IVDs)
service related FAQ’s
Based on the number of models and varients the timeline may change. usually the timeline for class B is approx 8-10 montsh and Class D is 14 months.

